Products
Highly sensitive COVID-19 Rapid Antigen Test
Coronavirus Rapid Test Cassette SARS-CoV-2 Ag Antigen Assay Kit
Manufacturer company WATMIND, approved and available in the list of the European Union and the Ministry of Health.
- Rapid coronavirus antigen test for nasopharyngeal and oropharyngeal secretions;
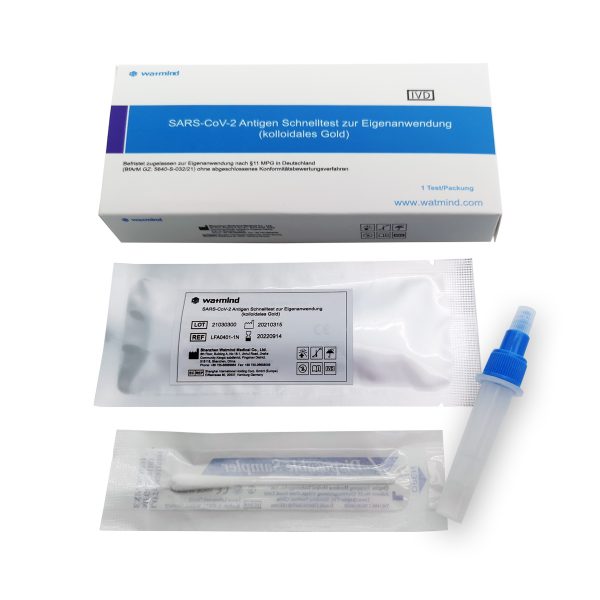
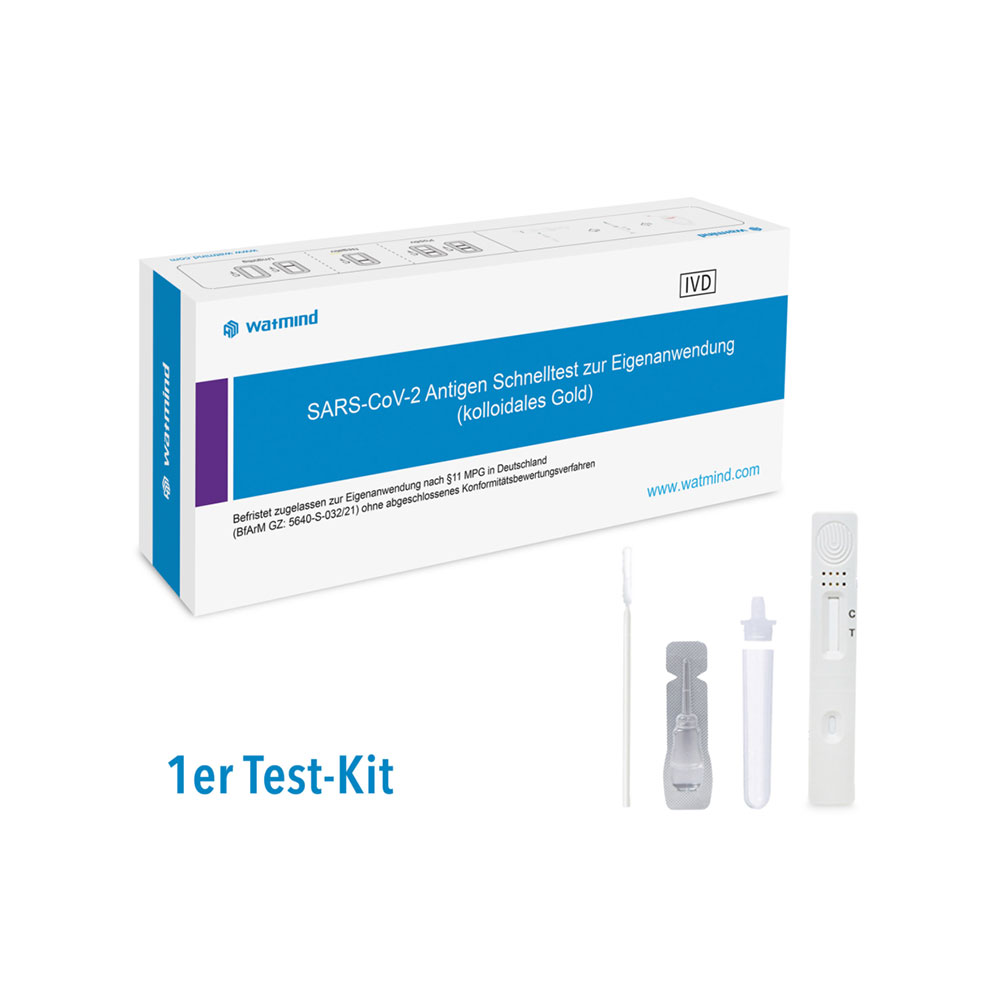
- Each package contains everything needed to perform the tests;
- Each test has a separate diluent;
- Available quality certificates and CE mark.
Principle:
Coronavirus antigen test (SARS-Cov-2) (tampon) is by immunochromatographic membrane analysis, which uses highly sensitive monoclonal antibodies to coronavirus.
The test consists of the following three parts, namely – sample pad, reagent pad and reaction membrane. The whole tape is fixed in a plastic device. The reactive membrane contains colloidal gold and embedded monoclonal antibodies against coronavirus, the reaction membrane contains secondary antibodies to coronavirus and polyclonal antibodies against murine globulin, which are pre-installed on the membrane.
When the sample is added to the test window, conjugates, which are contained in the reagent support are dissolved and combined with the sample. If coronavirus is present in the sample, the complex, which is formed between the anti-coronavirus conjugate will be captured by the specific anti-coronavirus monoclonal agent, marked with in T in the test cassette.
Whether the sample contains the virus or not, it continues to move to another reagent (anti-mouse IgG antibody), thus connecting the other conjugates, showing a red line in area C.
The new Antigen Rapid Test is an in vitro diagnostic test for the qualitative detection of new antigens in a nasal swab and nasal aspirate samples., using the rapid immunochromatographic method. Identification is based on monoclonal antibodies, specific for the new antigen.
Contents:
- Test cassette
- Sterilized nasopharyngeal / oropharyngeal swab
- Test tube
- Cap
- Ampoule buffer
- Instructions for use in Bulgarian
There is a separate diluent for each test.
Note: As with all diagnostic tests, the final clinical diagnosis should not be based on the result of a single test, and should only be prescribed by a physician after all clinical and laboratory results have been evaluated.